
Name: Dieter Riethmacher
Email: dieter.riethmacher@nu.edu.kz
Department of Biomedical Sciences
Research interest
During my career I have evolved from a pure developmental biologist to a researcher that tries to apply fundamental biological processes present during development to the more clinically relevant questions of disease development and progression. In recent years my interests have been spread from nerve degeneration and regeneration, inflammatory bowel disease (IBD) and cancer. In our group we are currently analyzing different genes/proteins for their potential involvement in the development and progression of diseases.
-
Periostin is an extracellular matrix protein that has been shown to be involved in cancer development as well as inflammation. Several isoforms are differentially expressed in different tissues and possibly during disease development and or progression. We are using cell culture techniques as well as working with human tissue and animal models to elucidate the function of periostin in tumor progression. We have been working on colorectal cancer (CRC) for some time already and became recently more interested in bladder cancer (BC) and a possible interaction between periostin and AXL, a tyrosine kinase and marker for epithelial-mesenchymal transition (EMT), that is a hallmark for invasiveness and thus metastasis.
-
We are currently analyzing the roles and possible interactions between periostin and CCL5-CCR5 signaling in IBD. Modification of both signaling systems may have implications not only for IBD but also for CRC.
-
The microbiome has recently received more and more attention and there is increasing evidence that the microbiome also strongly influences IBD and bowel cancer or CRC. We aim to analyze and potentially modify commensal bacteria in order to ameliorate IBD or even protect from developing IBD. The modification of commensal bacteria could just be to change to existing gut microbiota or even genetically modify bacteria (here using the CCL5-CCR5 signaling system) to improve patient outcome in these settings.
-
Based on the importance of the microbiome a natural new angle is to look into the gut phageome. Together with collaborators we will look into the healthy gut phageome and possible changes in IBD patients.
We have collaborators inside and outside Kazakstan and will apart from human patient samples also involve mouse models and cell culture techniques.
Project(s) for intake 2021
1. Analyzing the roles of Periostin in bladder cancer
Bladder Cancer (BC) is the 7th most common cancer worldwide in men and the 17th most common cancer worldwide in women. Each year, approximately 110,500 men and 70,000 women are diagnosed with as new cases and 38,200 patients in the European Union and 17,000 US patients die from BC [1]. Despite its prevalence, morbidity, mortality and associated cost of management, BC remains grossly under-recognized as a public health concern and underfunded scientifically [2]. The vast majority (≈ 70-75%) of as newly diagnosed bladder cancers are non-invasive (NMIBC) and often diagnosed with stages Ta, T1, or carcinoma in situ [3]. However, the remaining present with muscle-invasive disease (MIBC) with stages T2–4, associated with a high risk of death from distant metastases. Yet between 50 and 70% of NMIBC tumors do recur, and approximately 10–20% of them progress to muscle-invasive disease [3]. Periostin is a matricellular protein originally isolated from osteoblasts and was initially found to be preferentially expressed in the periosteum [4, 5]. Periostin has been shown to be up-regulated in a variety of different tumors among them bladder cancer [6]. In general high expression of periostin is linked to poor prognosis [7]. Periostin has been shown to have a variety of different functions in tumor progression including epithelial-mesenchymal transition, niche formation, tumor survival and angiogenesis [8-11]. To understand the general function of periostin several groups, including ours, have independently generated periostin-deficient animals [12-15]. As a secreted molecule and being present in EVs Periostin is in the ideal position to influence the tumor cells and the microenvironment in a paracrine and autocrine fashion. In a recent paper Ma et al. have shown that Periostin contributes to chemically induced colorectal tumorigenesis via paracrine integrin signaling. Initiated from the environment (cancer-associated fibroblast) producing Periostin and cancer cells reacting to it and signaling back to the environment [11, 16].
There are several BC cell lines available (human and murine) that will be analyzed for the level of Periostin expression on mRNA and protein level. Due to its high potency to induce bladder cancer, N-butyl-N-(4-hydroxybutyl)nitrosamine (BBN) is the most suitable reagent to generate chemically induced in vivo models of bladder cancer for studying bladder carcinogenesis [17]. We will use BBN to induce BC in our periostin-deficient animals and in controls. Using this strategy we will be able to address several important questions that will shed light on the roles of periostin in the development and progression of bladder cancer.
- will BBN induce BC in periostin-deficient animals to the same extend as in wildtype animals?
- will the induced BC lacking periostin be different regarding morphology and behavior (grade)?
- will wildtype and periostin-deficient cells behave similar regarding culture efficiency, proliferation rates and 3D structure and histology?
- will reintroduction of periostin (ideally the major splice variant expressed in theses cells) into periostin-deficient cells make these cells and cultures change their behavior ( similar or identical to wildtype cells)?
- we will also aim to label the cells with GFP (wildtype, periostin-deficient and rescued).
- In the third phase these cells will be transplanted back into mice.
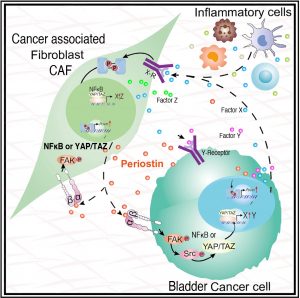 Figure 1: Model of the multiple paracrine and autocrine crosstalks between tumors cells and microenvironment (modified from Ma et al. 2020 Ref. 11). Please note that Periostin can be expressed in the cancer cells as well as the cancer associated fibroblasts. Periostin signaling is mediated via integrins and focal adhesion kinase (FAK) but then can use several downstream mediators like src-YAP/TAZ but also PI3Kinase and NFκB. Depending on downstream mediators different genes will be induced including virtual factors XYZ and periostin itself. The presence of these induced molecules in the microenvironment will then contribute to further signaling in an autocrine and a paracrine fashion. Our chemically induced tumor model will enable us to thoroughly dissect this signaling and understand which sources of Periostin are required at what stage of the disease.
Figure 1: Model of the multiple paracrine and autocrine crosstalks between tumors cells and microenvironment (modified from Ma et al. 2020 Ref. 11). Please note that Periostin can be expressed in the cancer cells as well as the cancer associated fibroblasts. Periostin signaling is mediated via integrins and focal adhesion kinase (FAK) but then can use several downstream mediators like src-YAP/TAZ but also PI3Kinase and NFκB. Depending on downstream mediators different genes will be induced including virtual factors XYZ and periostin itself. The presence of these induced molecules in the microenvironment will then contribute to further signaling in an autocrine and a paracrine fashion. Our chemically induced tumor model will enable us to thoroughly dissect this signaling and understand which sources of Periostin are required at what stage of the disease.
The research in this application will generate data that enable us to better understand the development and progression of bladder cancer (BC), in particular in relation to periostin.
- Burger, M., et al., Epidemiology and risk factors of urothelial bladder cancer. Eur Urol, 2013. 63(2): p. 234-41.
- Kaplan, A.L., M.S. Litwin, and K. Chamie, The future of bladder cancer care in the USA. Nat Rev Urol, 2014. 11(1): p. 59-62.
- Goonewardene, S.S., et al., Management of Non-Muscle Invasive Bladder Cancer. 2020: Springer International Publishing. 402.
- Horiuchi, K., et al., Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res, 1999. 14(7): p. 1239-49.
- Takeshita, S., et al., Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J, 1993. 294 ( Pt 1): p. 271-8.
- Gonzalez-Gonzalez, L. and J. Alonso, Periostin: A Matricellular Protein With Multiple Functions in Cancer Development and Progression. Front Oncol, 2018. 8: p. 225.
- Jang, S.Y., et al., The Combination of Periostin Overexpression and Microvascular Invasion Is Related to a Poor Prognosis for Hepatocellular Carcinoma. Gut Liver, 2016. 10(6): p. 948-954.
- Chen, L., et al., Periostin mediates epithelial-mesenchymal transition through the MAPK/ERK pathway in hepatoblastoma. Cancer Biol Med, 2019. 16(1): p. 89-100.
- Chuanyu, S., et al., Periostin promotes migration and invasion of renal cell carcinoma through the integrin/focal adhesion kinase/c-Jun N-terminal kinase pathway. Tumour Biol, 2017. 39(4): p. 1010428317694549.
- Liu, G.X., et al., Role of periostin and its antagonist PNDA-3 in gastric cancer metastasis. World J Gastroenterol, 2015. 21(9): p. 2605-13.
- Ma, H., et al., Periostin Promotes Colorectal Tumorigenesis through Integrin-FAK-Src Pathway-Mediated YAP/TAZ Activation. Cell Rep, 2020. 30(3): p. 793-806 e6.
- Rios, H., et al., periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol Cell Biol, 2005. 25(24): p. 11131-44.
- Brodarac, A., Impaired tooth development in periostin deficient mice., in ZMNH. 2006, University Hamburg: Hamburg.
- Kii, I., et al., Periostin is an extracellular matrix protein required for eruption of incisors in mice. Biochem Biophys Res Commun, 2006. 342(3): p. 766-72.
- Oka, T., et al., Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res, 2007. 101(3): p. 313-21.
- Silvers, C.R., et al., Identification of extracellular vesicle-borne periostin as a feature of muscle-invasive bladder cancer. Oncotarget, 2016. 7(17): p. 23335-45.
- Oliveira, P.A., et al., The N-butyl-N-4-hydroxybutyl Nitrosamine Mouse Urinary Bladder Cancer Model. Methods Mol Biol, 2018. 1655: p. 155-167.
2. Provide genetically modified bacterial strains that influence cancer development and progression.
In our group project, we want to identify new treatment alternatives in order to develop personalized cancer treatment. This project will build on the facts that the CCL5/CCR5 axis is involved in various processes of cancer including angiogenesis, tumor cell invasion and proliferation, recruitment of immune cells and re-sensitization to chemotherapy. It has been demonstrated that CCL5 and CCR5 are upregulated in the tumor microenvironment. A potent CCR5 antagonist, Maraviroc (initially developed as HIV-1 inhibitor) has been used to reduce tumor burden in animal models. We have developed a CCL5 derivative and antagonist of CCR5 that is way more potent than Maraviroc in HIV inhibition. We will test this antagonist by expressing it in specific bacterial strains in different mouse cancer models to analyze the effect on tumor development and progression. We expect that our antagonist, in combination with different bacteria, will inhibit tumor growth and/or progression. This could lead to a more personalized treatment for different cancers.
Cancer is one of the leading causes of mortality worldwide. An estimated 14 million new cases of cancer occur worldwide every year with numbers rising over the last decades [1]. Two of the most common malignancies are colorectal and gastric cancer, affecting each year around 1.2 and 0.5 million people, respectively [2, 3]. The first line of treatment is surgery with additional chemotherapy and radiotherapy. Colorectal cancer has a poor prognosis, with 40 % of patients dying in the first 5 years after diagnosis. Metastases are found in about 20% of the patients being diagnosed with colorectal cancer and develop in about 50% of patients in the course of the disease [4, 5]. Most patients with gastric cancer are only diagnosed at advanced stages of the disease [6]. Recurrence affects 50% of patients with a 5-year survival rate of around 20% [7]. So far, no specific treatment has been developed for these cancers. It is therefore essential to establish new treatments as well as earlier diagnosis methods to increase the chances of survival for people afflicted by these diseases [8, 9]. The CCL5/CCR5 axis plays a crucial role in the development a large number of tumors, with a steadily growing body of literature [10]. Due to a complex interplay of the CCL5/CCR5 axis in tumor progression and depending on the presence or absence of CCR5 expression on the tumor cells, receptor antagonism or agonism is the respective choice to tackle tumor development [11-15]. The group of Dr. Vangelista developed extremely potent CCL5-based CCR5 agonists and antagonists [16, 17]. When tested in vitro as HIV blockers, these CCL5 derivatives outclassed maraviroc anti-HIV potency by a 1000 fold, and their active secretion by engineered lactobacilli has already been proven successful [16, 18-20], thus providing the basis for our approach. Over the last years, it has been shown that commensal bacteria have an important effect on the immune system by promoting the development of tumor-specific T-cells [21-24]. The immune system is heavily involved in tumor development and progression, hence it is not surprising that the microbiota can have an effect on these processes [24]. In our project, we wish to combine two anti-tumor approaches by expressing our potent CCL5-based CCR5 agonist or antagonist (depending on the tumor type) in different bacterial strains (possibly using or flanking those strains that presented an anti-tumor effect) to analyze the in vivo effect on tumor development and progression. The research group working on this project will consist of Dr. D. Riethmacher, Dr. Vangelista, Dr. E. Riethmacher, Dr. D. Bulanin and Dr. E. Tulchinsky.
References
- Ferlay, J., I. Soerjomataram, R. Dikshit, S. Eser, C. Mathers, M. Rebelo, D.M. Parkin, D. Forman, and F. Bray, Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer, 2015. 136(5): p. E359-86.
- Ferlay, J., D.M. Parkin, and E. Steliarova-Foucher, Estimates of cancer incidence and mortality in Europe in 2008. European journal of cancer (Oxford, England : 1990), 2010. 46(4): p. 765-81.
- Jemal, A., F. Bray, M.M. Center, J. Ferlay, E. Ward, and D. Forman, Global cancer statistics. CA: a cancer journal for clinicians, 2011. 61(2): p. 69-90.
- Kamiyama, H., H. Noda, F. Konishi, and T. Rikiyama, Molecular biomarkers for the detection of metastatic colorectal cancer cells. World journal of gastroenterology, 2014. 20(27): p. 8928-38.
- Siegel, R., C. Desantis, and A. Jemal, Colorectal cancer statistics, 2014. CA: a cancer journal for clinicians, 2014. 64(2): p. 104-17.
- Hartgrink, H.H., E.P.M. Jansen, N.C.T. van Grieken, and C.J.H. van de Velde, Gastric cancer. Lancet (London, England), 2009. 374(9688): p. 477-90.
- Tsugane, S. and S. Sasazuki, Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association, 2007. 10(2): p. 75-83.
- Li, Y.-H., Y.-B. Niu, Y. Sun, F. Zhang, C.-X. Liu, L. Fan, and Q.-B. Mei, Role of phytochemicals in colorectal cancer prevention. World journal of gastroenterology, 2015. 21(31): p. 9262-72.
- Liu, Y., X. Zhang, C. Han, G. Wan, X. Huang, C. Ivan, D. Jiang, C. Rodriguez-Aguayo, G. Lopez-Berestein, P.H. Rao, D.M. Maru, A. Pahl, X. He, A.K. Sood, L.M. Ellis, J. Anderl, and X. Lu, TP53 loss creates therapeutic vulnerability incolorectal cancer. Nature, 2015. 520(7549): p. 697-701.
- Vangelista, L. and S. Vento, The Expanding Therapeutic Perspective of CCR5 Blockade. Frontiers in immunology, 2017. 8: p. 1981.
- Bronte, V. and E. Bria, Interfering with CCL5/CCR5 at the Tumor-Stroma Interface. Cancer cell, 2016. 29(4): p. 437-439.
- Gonzalez-Martin, A., E. Mira, and S. Manes, CCR5 in cancer immunotherapy: More than an "attractive" receptor for T cells. Oncoimmunology, 2012. 1(1): p. 106-108.
- Gonzalez-Martin, A., E. Mira, and S. Manes, CCR5 as a potential target in cancer therapy: inhibition or stimulation? Anti-cancer agents in medicinal chemistry, 2012. 12(9): p. 1045-57.
- Lapteva, N. and X.F. Huang, CCL5 as an adjuvant for cancer immunotherapy. Expert opinion on biological therapy, 2010. 10(5): p. 725-33.
- Velasco-Velazquez, M., W. Xolalpa, and R.G. Pestell, The potential to target CCL5/CCR5 in breast cancer. Expert opinion on therapeutic targets, 2014. 18(11): p. 1265-75.
- Secchi, M., V. Grampa, and L. Vangelista, Rational CCL5 mutagenesis integration in a lactobacilli platform generates extremely potent HIV-1 blockers. Scientific reports, 2018. 8(1): p. 1890.
- Vangelista, L., M. Secchi, and P. Lusso, Rational design of novel HIV-1 entry inhibitors by RANTES engineering. Vaccine, 2008. 26(24): p. 3008-15.
- Secchi, M., L. Vassena, S. Morin, D. Schols, and L. Vangelista, Combination of the CCL5-derived peptide R4.0 with different HIV-1 blockers reveals wide target compatibility and synergic cobinding to CCR5. Antimicrobial agents and chemotherapy, 2014. 58(10): p. 6215-23.
- Secchi, M., Q. Xu, P. Lusso, and L. Vangelista, The superior folding of a RANTES analogue expressed in lactobacilli as compared to mammalian cells reveals a promising system to screen new RANTES mutants. Protein expression and purification, 2009. 68(1): p. 34-41.
- Vangelista, L., M. Secchi, X. Liu, A. Bachi, L. Jia, Q. Xu, and P. Lusso, Engineering of Lactobacillus jensenii to secrete RANTES and a CCR5 antagonist analogue as live HIV-1 blockers. Antimicrobial agents and chemotherapy, 2010. 54(7): p. 2994-3001.
- Atarashi, K., T. Tanoue, M. Ando, N. Kamada, Y. Nagano, S. Narushima, W. Suda, A. Imaoka, H. Setoyama, T. Nagamori, E. Ishikawa, T. Shima, T. Hara, S. Kado, T. Jinnohara, H. Ohno, T. Kondo, K. Toyooka, E. Watanabe, S.-I. Yokoyama, S. Tokoro, H. Mori, Y. Noguchi, H. Morita, I.I. Ivanov, T. Sugiyama, G. Nunez, J.G. Camp, M. Hattori, Y. Umesaki, and K. Honda, Th17 Cell Induction by Adhesion of Microbes to Intestinal Epithelial Cells. Cell, 2015. 163(2): p. 367-80.
- Atarashi, K., T. Tanoue, K. Oshima, W. Suda, Y. Nagano, H. Nishikawa, S. Fukuda, T. Saito, S. Narushima, K. Hase, S. Kim, J.V. Fritz, P. Wilmes, S. Ueha, K. Matsushima, H. Ohno, B. Olle, S. Sakaguchi, T. Taniguchi, H. Morita, M. Hattori, and K. Honda, Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature, 2013. 500(7461): p. 232-6.
- Sefik, E., N. Geva-Zatorsky, S. Oh, L. Konnikova, D. Zemmour, A.M. McGuire, D. Burzyn, A. Ortiz-Lopez, M. Lobera, J. Yang, S. Ghosh, A. Earl, S.B. Snapper, R. Jupp, D. Kasper, D. Mathis, and C. Benoist, MUCOSAL IMMUNOLOGY. Individual intestinal symbionts induce a distinct population of RORgamma⁺ regulatory T cells. Science (New York, N Y ), 2015. 349(6251): p. 993-7.
- Tanoue, T., S. Morita, D.R. Plichta, A.N. Skelly, W. Suda, Y. Sugiura, S. Narushima, H. Vlamakis, I. Motoo, K. Sugita, A. Shiota, K. Takeshita, K. Yasuma-Mitobe, D. Riethmacher, T. Kaisho, J.M. Norman, D. Mucida, M. Suematsu, T. Yaguchi, V. Bucci, T. Inoue, Y. Kawakami, B. Olle, B. Roberts, M. Hattori, R.J. Xavier, K. Atarashi, and K. Honda, A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature, 2019. 565(7741): p. 600-605.




